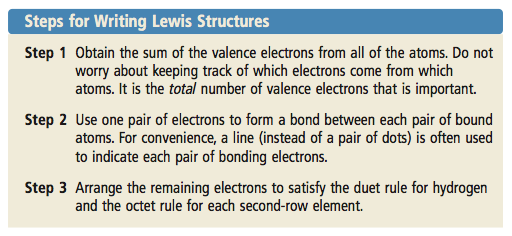
Central atom N. Dont be concerned with which atom gave what.

How To Draw Lewis Structures A Step By Step Tutorial Middle School Science Blog
H2O Lewis structure NH 3 Lewis structure Step 01.
. Lewis structure of O 3 can be drawn by starting from valence electrons of oxygen atoms in several steps. In the example O only has 1 particle so it is placed in the center. Place the steps for drawing a Lewis structure in the correct order.
Key Points To Consider When Drawing The Cl2 Electron Dot Structure. Because nitrogen trifluoride is a simple molecule these steps are not complex and do not. You have to follow few steps to draw the lewis structure of NF 3.
How to Draw a Lewis Dot Structure Step 1. The video covers the basic Lewis structures youll see in an introductory chemistry class. In the lewis structure of NH 3 you can see there are one double bond and one single.
Mark lone pairs Step 3. Heres how you can draw the BeF 2 lewis structure step by step. Follow these simple steps to correctly draw a Lewis dot structure.
Following steps are followed to draw lewis structures. Here are the steps that I use to draw a Lewis structure. Place least electronegative element in center and draw single bonds from the central atom to other atoms.
Each step of drawing the lewis structure of O 3 is explained in detail in this tutorial. Subtract electrons for positively charged ions. Put remaining valence electrons as lone pairs.
Draw the simple structure skeleton structure of the compound by connecting everything with single bonds only. Put any unused electrons on the central atom. 2 - Add the valence electrons contributed by each individual atom to determine the total number of outer electrons for the species.
Flexible Online Learning at Your Own Pace. Use two valence electrons to form each bond in the skeleton structure. For all but the simplest molecules the following step-by-step process is faster.
Sum the valence electrons from all the atoms. O 3 lewis structure. Find the Total Number of Valence Electrons.
If the entire molecule is charged ie. Add electrons to all the noncentral atoms. The SUM TOTAL is what is important.
Add up the total number of valence electrons found in the entire compound. Placing the Elements in the Drawing. Count the total number of valence electrons.
For the molecule under consideration calculate the total number of valence electrons for this add up the valency of all the atoms in the molecule. In the lewis structure of NF 3 there are three N-F bonds and one lone pair on nitrogen atom which is the center atom. Invest 2-3 Hours A Week Advance Your Career.
Drawing Lewis Structures In short these are the steps you need to follow for drawing a Lewis structure. How to Draw Lewis Structures Step 1. Group number for each element of valence electrons.
Determine the total number of valence electrons to be depicted in the Lewis diagram. Each fluorine atom has three lone pairs. Determine the total number of valence electrons.
Generally electrons are paired. Decide which atom is the central atom in the structure. Find the Total Number of Valence Electrons refer to the Instructions Below the Pictures.
Following are the steps to construct the Lewis Structure. Ad Build your Career in Data Science Web Development Marketing More. Drawing Lewis Structures A step-by-step Guide by C.
Steps of drawing lewis structure of NF 3. Steps for drawing a Lewis Structurepdf - STEPS FOR DRAWING A LEWIS STRUCTURE. The first step is to sketch the Lewis structure of the Cl2 molecule to add valence electrons around the two chlorine atoms and the final step is to combine the two chlorine diatomic atoms to get the Cl2 Lewis Structure.
The trial-and-error method for writing Lewis structures can be time consuming. Determine how many electrons must be added to central element. For an anionic molecule add one electron for one negative charge to the valency of the.
A video tutorial for how to draw Lewis Structures in five steps. Calculation of total valence electrons of NH3 Step 02. Instructions for Drawing Lewis Structures For molecules and polyatomic ions composed of nonmetals.
Turn lone pairs into bonds as each atom fulfill electron octet rule. Connect four atoms via single bonds. Step 3 Attach the terminal atoms using pairs of electrons Step 4 Fill the octet of the terminal atoms Step 5.
Write the correct skeletal structure for the molecule. Write the skeleton structure of the molecule. LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1.
NF 3 lewis structure. 1 - Count the number of outer electrons contributed by each atom. All valence electrons of the atoms in Lewis structures must be shown.
Hydrogen atoms are always terminal only one bond Put more electronegative elements in terminal positions 2. Draw a trial structure by putting electron pairs around every atom until each gets an octet. A three-step approach for drawing the Cl2 Lewis structure can be used.
A polyatomic anion or cation add. List of steps to drawing Lewis structures STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by jmurphy68 Terms in this set 6 Step 1 Calculate the valence electrons Step 2 Determine the central atom. Hoeger 1 Determine the total number of valence electrons for ALL atoms.
After drawing the lewis structure of NH 3 you can decide shape of the O 3 molecule. CO 2 Total 16 Step 2. Unpaired electrons are observed in odd electron molecules such as NO and NO2.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom. Starting with the first step at the top of the list. NH3 Lewis structure N 2 Lewis structure Step 01.
Draw sketch Step 2. This gives the total number of electrons for the Lewis structure. Count the valence electrons of atoms To draw Lewis structure we need to figure out the number of valence electrons in individual atoms as shown in the table below.
Add electrons for negatively charged ions. Count the valence electrons in your trial structure.

Lewis Diagrams Made Easy How To Draw Lewis Dot Structures Youtube
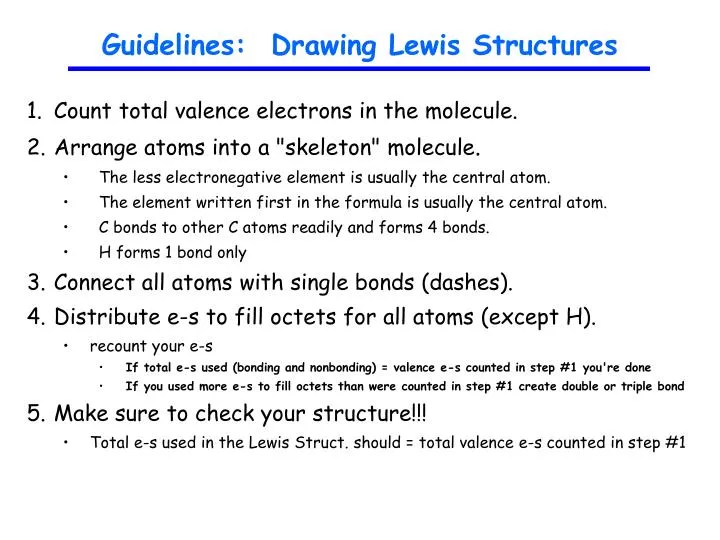
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Youtube

Lewis Structures 5 Steps 1 Count Valence E Available If An Anion Add Charge To Valence E If A Cation Subtract Charge From Valence E 2 Draw Skeleton Ppt Download

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421

Chemistry 101 Draw Lewis Dot Structures For Covalent Compounds Youtube

0 comments
Post a Comment